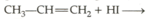Preparations of Haloalkanes
Preparations of Haloalkanes: Overview
This topic covers concepts, such as Preparations of Haloalkanes, Preparation of Haloalkanes From Alcohols, Preparation of Haloalkanes by Free Radical Halogenation of Alkanes, and Preparation of Haloalkanes by Addition of Halogen Acids to Alkenes.
Important Questions on Preparations of Haloalkanes
The major product of the following reaction is:
Which of the following is an example of Sandmeyer reaction:
(i)
(ii)
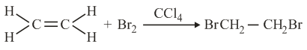
In the presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti-Markovnikoff’s addition with alkenes, because of the fact that:
Consider the following reaction
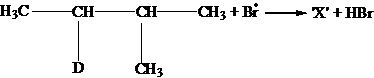
Identify the structure of the major product X.
The reaction condition leading to the best yield of are –
The reaction condition leading to the best yield of are
The following reaction is known as
Which of the following cannot be prepared by Sandmeyer's reaction?
Which of the following will not give haloderivative by addition of ?
Chlorobenzene is prepared commercially by
Chlorobenzene is formed by reaction of chlorine with benzene in the presence of . Which of the following species attacks the benzene ring in this reaction?
Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated at room temperature?
The reaction, , is known as
The reaction of toluene with chlorine in the presence of iron and in the absence of light yields _____.
What happens in Finkelstein reactions?
For the chemical reaction:
The IUPAC name of the product is:
Conversion of methyl chloride into methyl fluoride is known as
What are and in the following reaction:
Which one of the following hydrocarbons when treated with chlorine will give only, single monochloro compound?
Which of the following substrate is most reactive toward free radical substitution reaction :-